PDN Experimental Project
Modelling calcium feedback mechanisms in light adaptation in vertebrate cone photoreceptors
For my final year of undergraduate studies at Cambridge, I did an Experimental Project with Dr. Hugh Matthews in the Physiology, Development, and Neuroscience (PDN) Department on mathematical modelling of photocurrent in cone photoreceptors. Revealing how cones adapt to bright light illuminates the basis of daytime vision and informs the design of artificial photoreceptors and vision therapies. Although comprehensive models have been developed to describe rod phototransduction and adaptation under scotopic (low-light) conditions, these frameworks typically fail to account for the rapid gain adjustments and accelerated recovery kinetics characteristic of cones operating in the photopic (bright-light) region. Bridging this gap requires a minimal yet physiologically grounded model that incorporates cone-specific parameters and feedback loops.
If you’re interested in learning more, contact me for the full dissertation.
Background
In vertebrates, vision relies on photoreceptors, known as rods and cones, to detect light across varying intensities. Visual sensory information in the form of light is converted into electrical signals in a process known as phototransduction, consisting of a G protein-coupled signalling cascade
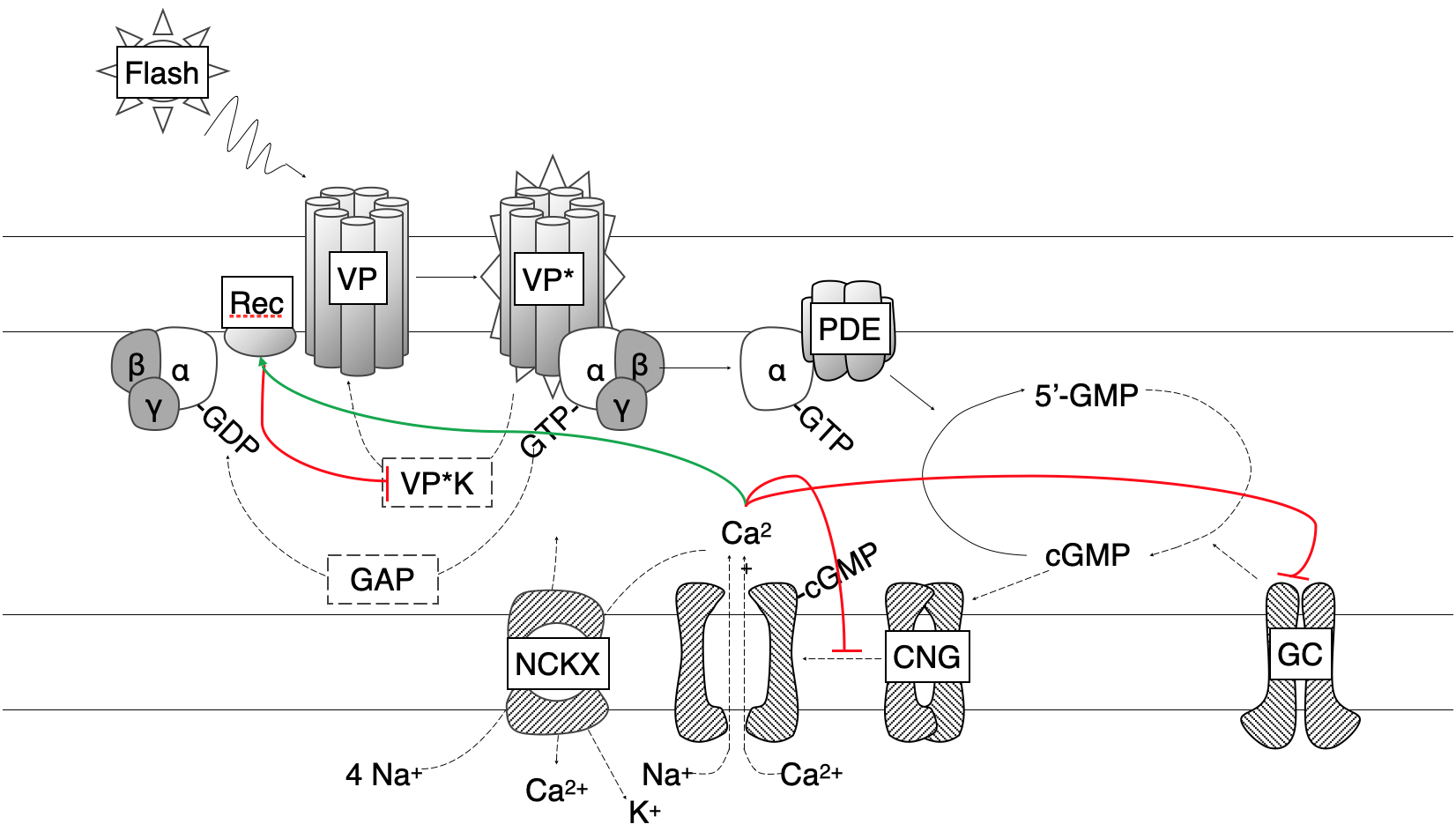
Phototransduction at Rest
In darkness, photoreceptors maintain a steady, or “dark,” current driven by continuous production of cyclic guanosine monophosphate (cGMP). Constitutively active guanylyl cyclase (GC) produces cGMP, which binds to cyclic nucleotide-gated (CNG) channels. Open CNG channels permit influx of Na⁺ and Ca²⁺, generating a depolarizing inward current. Calcium entering through these channels represents a fraction of the total dark current; it is extruded in exchange for Na⁺ and K⁺ by the Na⁺/Ca²⁺-K⁺ exchanger (NCKX), thus balancing intracellular Ca²⁺ levels and setting the photoreceptor’s resting operating point.
Activation of the Photoresponse
When photons of appropriate wavelength isomerize the chromophore in the visual pigment (VP), the opsin-bound pigment undergoes a conformational change to its active state (VP*). VP* acts as a G-protein-coupled receptor, binding transducin (T) and catalyzing the exchange of GDP for GTP on its α-subunit. The resulting Tα·GTP complex (T*) activates phosphodiesterase (PDE), which hydrolyzes cGMP. As cGMP concentration falls, CNG channels close, reducing the dark current and hyperpolarizing the cell.
Termination of the Photoresponse
To restore the dark-adapted condition, VP* and PDE* must be inactivated
Light Adaptation
Cones, which operate in bright light, must prevent saturation at increasing ambient light levels through a process called light adaptation. Light adaptation involves reduced response amplitude (lowering sensitivity) and faster kinetics (speeding up recovery). The amplitude reduction manifests as a smaller change in circulating current relative to the dark current; accelerated kinetics appear as a shortened time-to-peak and onset of recovery following a flash.
Calcium has been shown to be central in this process. During illumination, Ca²⁺ influx through residual open CNG channels decreases, while extrusion via NCKX continues, leading to a fall in intracellular Ca²⁺. This decline triggers three feedback pathways:
-
GCAP-Mediated GC Activation: Lower Ca²⁺ relieves inhibition of GC-activating proteins (GCAP1 and GCAP2), boosting GC activity to replenish cGMP more rapidly
. -
Modulation of CNG Channel Affinity: Ca²⁺-bound calmodulin (in rods) or CNG-modulin (in cones) alters channel cGMP affinity, decreasing sensitivity; this effect is modest in rods but may be amplified in cones
. -
Recoverin-Controlled VP Quenching: At high Ca²⁺, recoverin binds VPK, inhibiting pigment phosphorylation; falling Ca²⁺ releases recoverin, enhancing VPK activity, accelerating pigment inactivation, and shortening the light response
.
Collectively, these Ca²⁺-dependent loops reduce gain and accelerate recovery, expanding the dynamic range of photoreceptors to detect incremental light changes even under bright illumination.
Approach
Adaptating a Rod Framework
We began with a streamlined rod phototransduction model
Stay tuned for more (this was lengthier than I remembered)